Convenient synthesis of 6-alkyl phenanthridines and 1-alkyl isoquinolines via silver-catalyzed oxidative radical decarboxylation†
Abstract
A convenient and efficient protocol for the synthesis of 6-alkyl phenanthridines and 1-alkyl isoquinolines has been developed. The reaction relies on the coupling of 2-isocyanobiphenyls and vinyl isonitriles with alkyl radicals formed by the silver-catalyzed decarboxylation of stoichiometric aliphatic carboxylic acids, and affords diverse phenanthridine and isoquinoline derivatives under mild reaction conditions. The experiment of β-scission of cyclobutylcarbinyl radicals is used to shed light on the reaction mechanism.
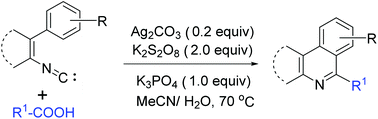


 Please wait while we load your content...
Please wait while we load your content...