Deconjugative alkylation/Heck reaction as a simple platform for dihydronaphthalene synthesis†
Abstract
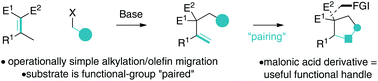
A simple platform for carbocycle synthesis by Knoevenagel adduct deconjugative alkylation/Heck reaction is described. Deconjugative alkylation of Knoevenagels adducts is two-fold synthetically enabling because C–C bond formation is (1) operationally simple due to the ease of Knoevenagel adduct carbanion generation and (2) results in alkene migration, which poises the substrate for cyclization. Furthermore, the gem-dinitrile moiety serves as a functional group for synthetic manipulation.

 Please wait while we load your content...
Please wait while we load your content...