Enantioselective synthesis of spirooxindole benzoquinolizines via organo-catalyzed cascade reactions†
Abstract
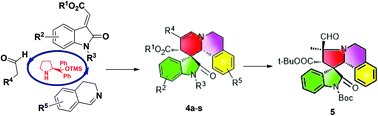
A Michael–Mannich–hemiaminalization–dehydration cascade reaction was developed for the construction of spirooxindole benzoquinolizine derivatives. Additionally, spirooxindole benzoindolizidine was prepared conveniently through a ring-contracted rearrangement reaction from spirooxindole benzoquinolizine.

 Please wait while we load your content...
Please wait while we load your content...