“On water” reaction of deactivated anilines with 4-methoxy-3-buten-2-one, an effective butynone surrogate†
Abstract
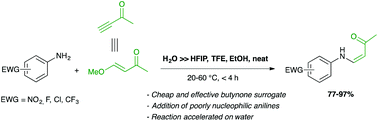
Poorly nucleophilic aromatic amines (nitroanilines, chloroanilines, etc.) react readily and selectively with trans-4-methoxy-3-buten-2-one, a convenient, effective and inexpensive surrogate for 3-butyn-2-one, to afford (Z)-enaminones. The efficiency of the reaction mostly lies in the use of water as a solvent, which enhances the reaction rate by a 45 to 200-fold factor with regard to other media.


 Please wait while we load your content...
Please wait while we load your content...