A rapid and clean synthetic approach to cyclic peptides via micro-flow peptide chain elongation and photochemical cyclization: synthesis of a cyclic RGD peptide†
Abstract
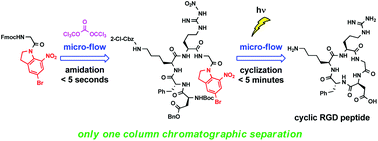
A cyclic RGD peptide was efficiently synthesized based on micro-flow, triphosgene-mediated peptide chain elongation and micro-flow photochemical macrolactamization. Our approach enabled a rapid (amidation for peptide chain elongation <5 s, macrolactamization <5 min) and clean (only one column chromatographic separation) synthesis of a cyclic peptide.

 Please wait while we load your content...
Please wait while we load your content...