One pot synthesis of α-ketoamides from ethylarenes and amines: a metal free difunctionalization strategy†
Abstract
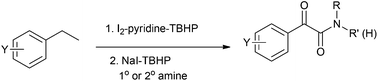
One-pot and metal free synthesis of α-ketoamides has been described through in situ generation of aryl ketones from easily available ethylarenes followed by amidation with various amines. This multiple oxidation protocol involves catalytic I2–pyridine–TBHP (t-butyl hydroperoxide) mediated oxidative benzylic carbonylation and sequential NaI–TBHP mediated oxidative amidation without using any solvent.

 Please wait while we load your content...
Please wait while we load your content...