α-Methylphenacyl thioesters as convenient thioacid precursors†
Abstract
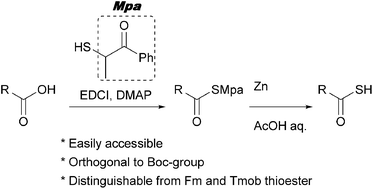
α-Methylphenacyl (Mpa) thioesters are described as precursors of thioacids. Mpa thioesters are accessible via the condensation of carboxylic acids and phenacyl thiol, which is easily prepared without column chromatography. The Mpa thioesters are selectively deprotected by reduction with zinc dust in the presence of conventional thioacid protecting groups. In addition, the Mpa group exhibits orthogonal reactivity to the Boc group. These features are expected to facilitate the preparation of complex thioacids, including those in peptides.

 Please wait while we load your content...
Please wait while we load your content...