Catalytic enantioselective acyl transfer: the case for 4-PPY with a C-3 carboxamide peptide auxiliary based on synthesis and modelling studies†
Abstract
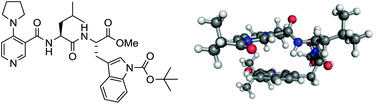
A series of 4-pyrrolidinopyridine (4-PPY) C-3 carboxamides containing peptide-based side chains have been synthesised and evaluated in the kinetic resolution of a small library of chiral benzylic secondary alcohols. A key design element was the incorporation of a tryptophan residue in the peptide side chain for promoting π-stacking between peptide side chain and the pyridinium ring of the N-acyl intermediate, in which modelling was used as a structure-based guiding tool. Together, a catalyst containing a LeuTrp-N-Boc side chain (catalyst 8) was identified that achieved s-values up to and in slight excess of 10. A transition-state model based on the modelling is proposed to explain the origin of enantioselectivity. This study establishes the usefulness of modelling as a structure-based guiding tool for enantioselectivity optimization as well as the potential for developing scalable peptide-based DMAP-type catalysts for large-scale resolution work.


 Please wait while we load your content...
Please wait while we load your content...