Selective complexation of divalent cations by a cyclic α,β-peptoid hexamer: a spectroscopic and computational study†
Abstract
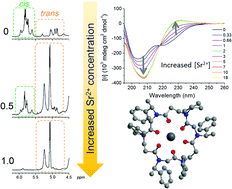
We describe the qualitative and quantitative analysis of the complexation properties towards cations of a cyclic peptoid hexamer composed of alternating α- and β-peptoid monomers, which bear exclusively chiral (S)-phenylethyl side chains (spe) that have no noticeable chelating properties. The binding of a series of monovalent and divalent cations was assessed by 1H NMR, circular dichroism, fluorescence and molecular modelling. In contrast to previous studies on cations binding by 18-membered α-cyclopeptoid hexamers, the 21-membered cyclopeptoid cP1 did not complex monovalent cations (Na+, K+, Ag+) but showed selectivity for divalent cations (Ca2+, Ba2+, Sr2+ and Mg2+). Hexacoordinated C-3 symmetrical complexes were demonstrated for divalent cations with ionic radii around 1 Å (Ca2+ and Ba2+), while 5-coordination is preferred for divalent cations with larger (Ba2+) or smaller ionic radii (Mg2+).

 Please wait while we load your content...
Please wait while we load your content...