Pd-Catalyzed C(sp3)–C(sp2) cross-coupling of Y(CH2SiMe3)3(THF)2 with vinyl bromides and triflates†
Abstract
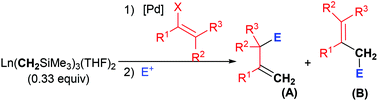
Pd-Catalyzed C(sp3)–C(sp2) cross-coupling of Y(CH2SiMe3)3(THF)2 with vinyl bromides and triflates has been developed for efficient synthesis of various allyltrimethylsilanes. The cross-coupling reaction was conducted at room temperature with low catalyst loading of either Pd(PPh3)4 or Pd(PPh3)2Cl2, and exhibited high efficiency and a broad substrate scope. In combination with the cross-coupling by the Lewis-acid catalyzed Hosomi–Sakurai reaction, a novel three-component one-pot cascade reaction was then accomplished to deliver homoallylic alcohols and ethers with high regioselectivity and diastereoselectivity. The three-component reaction defined the yttrium complex as a novel one-carbon synthon, which could either trigger bifunctionalization of alkenes or link two electrophiles and would find applications in organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...