One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody–drug conjugates†
Abstract
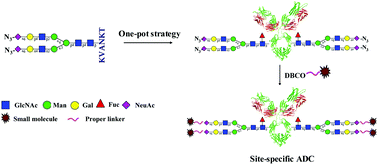
Chemoenzymatic transglycosylation catalyzed by endo-S mutants is a powerful tool for in vitro glycoengineering of therapeutic antibodies. In this paper, we report a one-pot chemoenzymatic synthesis of glycoengineered Herceptin using an egg-yolk sialylglycopeptide (SGP) substrate. Combining this one-pot strategy with novel non-natural SGP derivatives carrying azido or alkyne tags, glycosite-specific conjugation was enabled for the development of new antibody–drug conjugates (ADCs). The site-specific ADCs and semi-site-specific dual-drug ADCs were successfully achieved and characterized with SDS-PAGE, intact antibody or ADC mass spectrometry analysis, and PNGase-F digestion analysis. Cancer cell cytotoxicity assay revealed that small-molecule drug release of these ADCs relied on the cleavable Val-Cit linker fragment embedded in the structure. These results represent a new approach for glycosite-specific and dual-drug ADC design and rapid synthesis, and also provide the structural requirement for their biologic activities.

 Please wait while we load your content...
Please wait while we load your content...