Total synthesis of odoamide, a novel cyclic depsipeptide, from an Okinawan marine cyanobacterium†
Abstract
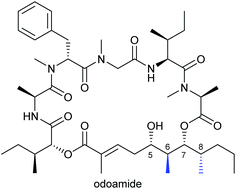
Odoamide is a novel cyclic depsipeptide with highly potent cytotoxic activity isolated from the Okinawan marine cyanobacterium Okeania sp. It contains a 26-membered macrocycle composed of a fatty acid moiety, a peptide segment and isoleucic acid. Four possible stereoisomers of the odoamide polyketide substructure were synthesised using a chiral pool approach. The first total synthesis of odoamide was also successfully achieved. The structure of synthetic odoamide was verified by comparing its NMR spectra with those of the natural product.


 Please wait while we load your content...
Please wait while we load your content...