Modular access to vicinally functionalized allylic (thio)morpholinonates and piperidinonates by substrate-controlled annulation of 1,3-azadienes with hexacyclic anhydrides†‡
Abstract
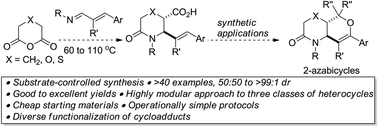
A modular substrate-controlled hexannulation of inherently promiscuous 1,3-azadienes with hexacyclic anhydrides, which affords versatile vicinally functionalized allylic lactams, in high yields, regio- and stereoselectivities is described.

 Please wait while we load your content...
Please wait while we load your content...