Tin powder-promoted one-pot synthesis of 3-spiro-fused or 3,3′-disubstituted 2-oxindoles†
Abstract
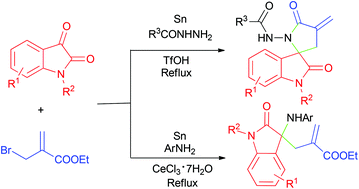
A convenient and efficient method for the constructions of 3-spirooxindole derivatives or 3,3′-disubstituted oxindoles has been developed from one-pot reactions of isatins, hydrazides or aromatic amines, 2-(bromomethyl)acrylic ester and tin powder in the presence of a catalytic amount of Brønsted or Lewis acid. The method avoids the use of toxic stannanes and allows easy operation. It is also proved that hydrazides are more favorable for intramolecular cyclization than amines.

 Please wait while we load your content...
Please wait while we load your content...