Elaboration of tetra-orthogonally-substituted aromatic scaffolds towards novel EGFR-kinase inhibitors†
Abstract
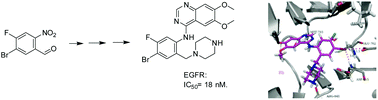
Nitration of three regioisomers of bromo-fluorobenzaldehyde proceeds regioselectively, notably with H2SO4/HNO3 at 0 °C. The thereby synthesized tetrasubstituted aromatics, endowed with orthogonal substituents, can be elaborated via Pd-catalysed coupling, reduction and reductive amination reactions. As a test-case, these compounds were converted into EGFR inhibitors related to Gefitinib, whose activity was rationalised by docking studies.


 Please wait while we load your content...
Please wait while we load your content...