Efficient and stereocontrolled synthesis of chondroitin mono- and disaccharide linked to variously sulfated biotinylated trisaccharides of the linkage region of proteoglycans†
Abstract
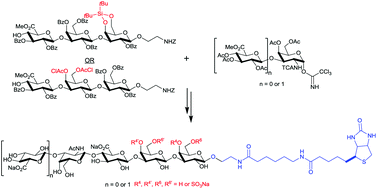
Efficient and stereocontrolled preparation of a library of variously sulfated biotinylated tetra- and pentasaccharides possessing the backbone of the partial linkage region plus the first chondroitin sulfate mono- or disaccharide unit (D-GlcA)n-β-D-(1,3)-GalNAc-β-D-(1,4)-GlcA-β-D-(1,3)-Gal-β-D-(1,3)-Gal (n = 0 or 1) is reported herein for the first time. The synthesis of these compounds was achieved using common key intermediates and a disaccharide building block obtained by semisynthesis. Stereoselective glycosylation, selective protection/deprotection steps, efficient reduction of the N-trichloroacetyl group into the corresponding N-acetyl group, efficient sulfation strategy, deprotection and biotinylation afforded target oligomers in good yield with high purity.

 Please wait while we load your content...
Please wait while we load your content...