Pd-catalyzed divergent trifluoroethylation and arylation of arylboronic acids by aryl(2,2,2-trifluoroethyl)iodonium triflates†
Abstract
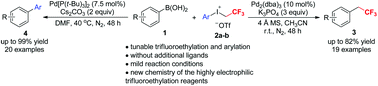
Highly electrophilic aryl(2,2,2-trifluoroethyl)iodonium triflates have been used for the first time as trifluoroethyl and aryl transfer reagents in Pd-catalyzed functionalization of arylboronic acids. Electron-rich arylboronic acids reacted with aryl(2,2,2-trifluoroethyl)iodonium triflates (2a–b) in CH3CN in the presence of Pd2(dba)3 and K3PO4 at room temperature to provide trifluoroethyl arenes in up to 82% yield, while the reactions of both electron-rich and -poor arylboronic acids with 2a–b in DMF in the presence of Pd[P(t-Bu)3]2 and Cs2CO3 at 40 °C afforded arylation products in up to 99% yield. This tunable protocol allows access to trifluoroethyl arenes or biaryls in good to excellent yields under mild conditions and without the addition of extra ligands.

 Please wait while we load your content...
Please wait while we load your content...