A direct assay of butyrylcholinesterase activity using a fluorescent substrate†
Abstract
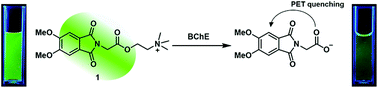
In this study, we report a direct fluorometric assay for butyrylcholinesterase (BChE) activity and screening of its inhibitor, using a fluorescent substrate. 2-(2-(5,6-Dimethoxy-1,3-dioxoisoindolin-2-yl)acetoxy)-N,N,N-trimethylethan-1-ammonium iodide (1) was hydrolyzed by BChE, and its fluorescence was quenched by an intramolecular photoinduced electron transfer process. The resulting change in fluorescence provided a facile method for real-time BChE activity testing. Remarkably, 1 was selectively hydrolyzed by BChE, even in the presence of excess acetylcholinesterase, thereby facilitating the specific monitoring of BChE activity. This assay method is also useful for screening potential BChE inhibitors. Given its simplicity, selectivity, and higher assay speed, this method may be extended to high-throughput screening of BChE inhibitors and relevant drug discovery.

 Please wait while we load your content...
Please wait while we load your content...