Organocatalytic vinylogous Mannich reaction of trimethylsiloxyfuran with isatin-derived benzhydryl-ketimines†
Abstract
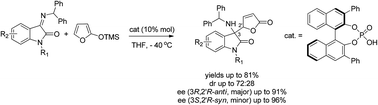
A family of chiral quaternary 3-aminooxindole butenolides has been synthesized by BINOL-derived phosphoric acid-catalyzed addition of trimethylsiloxyfuran to isatin-derived ketimines. Such a vinylogous Mannich-type reaction was found to produce diastereoisomeric butenolides in good yields and in most cases high enantiomeric excesses. The configurational assignment of the obtained products was safely performed by chemical correlation. A computational study of the transition state allowed rationalizing the obtained stereochemical outcome, highlighting the possible binding modes of the catalyst–imine–nucleophile transition complex.


 Please wait while we load your content...
Please wait while we load your content...