Synthesis of “neoprofen”, a rigidified analogue of ibuprofen, exemplifying synthetic methodology for altering the 3-D topology of pharmaceutical substances†
Abstract
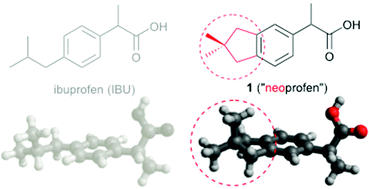
3,3-Dimethylcyclopentanes (neopentylenes) are ubiquitous in Nature but largely absent from synthetic pharmaceutical libraries. Neopentylenes define a hydrophobic and rigid 3-D topology with distinct molecular pharmacology, as exemplified here with two neopentylene-fused analogues of the synthetic anti-inflammatory drug, ibuprofen.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...