Nickel-catalyzed Chan–Lam cross-coupling: chemoselective N-arylation of 2-aminobenzimidazoles†
Abstract
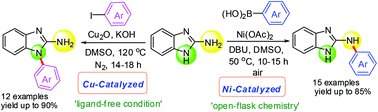
A complementary set of Ni- and Cu-based catalyst systems for the selective N-arylation of 2-aminobenzimidazoles have been developed. Selective N-arylation of the primary amine (C-NH2) group was achieved by Ni-catalyzed, boronic acid promoted cross-coupling reactions in air, whereas, selective N-arylation of the azole nitrogen was achieved with Cu-catalysis and aryl halides. These protocols are general and give rapid access to an array of both the N-arylated isomers of 2-aminobenzimidazoles.


 Please wait while we load your content...
Please wait while we load your content...