Copper-catalysed oxidative amination of quinoxalin-2(1H)-ones with aliphatic amines†
Abstract
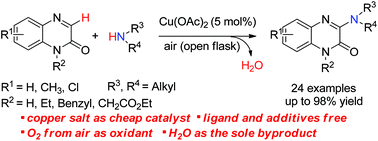
A novel, efficient and practical method for copper-catalysed oxidative C-3 amination of quinoxalin-2(1H)-ones with primary or secondary amines as the nitrogen sources has been developed. A wide variety of 3-aminoquinoxalin-2(1H)-ones were prepared in up to 98% yield with good functional group tolerance for 24 examples. This synthetic strategy features atom economy, concise steps, easy operation, and mild reaction conditions.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...