Brønsted acid-catalyzed regioselective reactions of 2-indolylmethanols with cyclic enaminone and anhydride leading to C3-functionalized indole derivatives†
Abstract
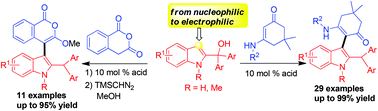
An abnormal regioselective substitution of 2-indolylmethanols with nucleophiles such as cyclic enaminone and cyclic anhydride has been established in the presence of Brønsted acid, which efficiently afforded C3-functionalized indole derivatives with structural diversity in high yield and regiospecificity (40 examples, up to 99% yield). Using this approach, the reactivity of the C3-position of the indole was switched from nucleophilic to electrophilic, which could serve as an “umpolung” strategy in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...