Direct carbon–carbon bond formation via reductive soft enolization: a syn-selective Mannich addition of α-iodo thioesters†
Abstract
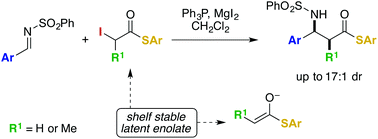
The β-amino carboxylic acid moiety is a key feature of numerous important biologically active compounds. We describe a syn-selective direct Mannich addition reaction that uses α-iodo thioesters and sulfonyl imines and produces β-amino thioesters. Enolate formation is achieved by reductive soft enolization. The products of the reaction provide straightforward access to biologically important β-lactams through a variety of known reactions.

 Please wait while we load your content...
Please wait while we load your content...