Synthesis of cyclopenta-fused polycyclic aromatic hydrocarbons utilizing aryl-substituted anilines†
Abstract
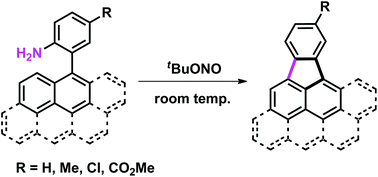
Cyclopenta-fused polycyclic aromatic hydrocarbons (CP-PAHs), potentially electronically and biologically highly active materials, were synthesized from readily available 2-aryl-substituted anilines. Reactions occur under extremely mild, room temperature conditions using tBuONO as the sole reagent. The use of a nitrite source generates a reactive diazonium intermediate in situ that then reacts with a tethered polycyclic aromatic moiety by intramolecular aromatic substitution. This protocol could be presented as one of the simplest methods to access CP-PAHs.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...