A novel bis(pinacolato)diboron-mediated N–O bond deoxygenative route to C6 benzotriazolyl purine nucleoside derivatives†
Abstract
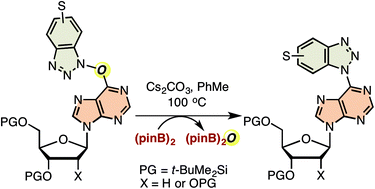
Reaction of amide bonds in t-butyldimethylsilyl-protected inosine, 2′-deoxyinosine, guanosine, 2′-deoxyguanosine, and 2-phenylinosine with commercially available peptide-coupling agents (benzotriazol-1H-yloxy)tris(dimethylaminophosphonium) hexafluorophosphate (BOP), (6-chloro-benzotriazol-1H-yloxy)trispyrrolidinophosphonium hexafluorophosphate (PyClocK), and (7-azabenzotriazol-1H-yloxy)trispyrrolidinophosphonium hexafluorophospate (PyAOP) gave the corresponding O6-(benzotriazol-1-yl) nucleoside analogues containing a C–O–N bond. Upon exposure to bis(pinacolato)diboron and base, the O6-(benzotriazol-1-yl) and O6-(6-chlorobenzotriazol-1-yl) purine nucleoside derivatives obtained from BOP and PyClocK, respectively, underwent N–O bond reduction and C–N bond formation, leading to the corresponding C6 benzotriazolyl purine nucleoside analogues. In contrast, the 7-azabenzotriazolyloxy purine nucleoside derivatives did not undergo efficient deoxygenation, but gave unsymmetrical nucleoside dimers instead. This is consistent with a prior report on the slow reduction of 1-hydroxy-1H-4-aza and 1-hydroxy-1H-7-azabenzotriazoles. Because of the limited number of commercial benzotriazole-based peptide coupling agents, and to show the applicability of the method when such coupling agents are unavailable, 1-hydroxy-1H-5,6-dichlorobenzotriazole was synthesized. Using this compound, silyl-protected inosine and 2′-deoxyinosine were converted to the O6-(5,6-dichlorobenzotriazol-1-yl) derivatives via in situ amide activation with PyBroP. The O6-(5,6-dichlorobenzotriazol-1-yl) purine nucleosides so obtained also underwent smooth reduction to afford the corresponding C6 5,6-dichlorobenzotriazolyl purine nucleoside derivatives. A total of 13 examples were studied with successful reactions occurring in 11 cases (the azabenzotriazole derivatives, mentioned above, being the only unreactive entities). To understand whether these reactions are intra or intermolecular processes, a crossover experiment was conducted. The results of this experiment as well as those from reactions conducted in the absence of bis(pinacolato)diboron and in the presence of water indicate that detachment of the benzotriazoloxy group from the nucleoside likely occurs, followed by reduction, and reattachment of the ensuing benzotriazole, leading to products.

 Please wait while we load your content...
Please wait while we load your content...