Regioselective routes to orthogonally-substituted aromatic MIDA boronates†
Abstract
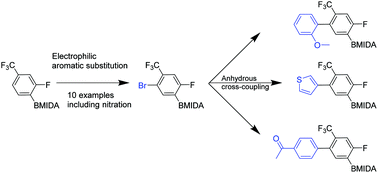
A series of tetrasubstituted aromatics has been synthesized, many of which are based on elaborated N-methyliminodiacetic acid (MIDA)-boronates. A sequence employing nitration, bromination, stepwise Suzuki–Miyaura (SM) coupling with a boronic acid, then base-mediated unmasking of the boronic acid from the MIDA-boronate and a second SM-coupling has led to our desired, mainly 1,2,4,5-substituted tetrasubstituted aromatic targets.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...