A rare γ-pyranopyrazole skeleton: design, one-pot synthesis and computational study†
Abstract
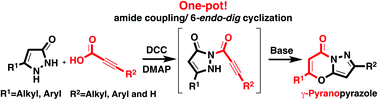
Drawing upon a consecutive amide coupling and intramolecular cyclisation pathway, a one-pot, straightforward synthetic route has been developed for a range of pyrazole fused γ-pyrone derivatives. The reaction mechanism proposed for the chemoselective formation of γ-pyranopyrazole is furthermore fully supported by experimental and computational studies.

 Please wait while we load your content...
Please wait while we load your content...