Synthesis and in vitro bone cell activity of analogues of the cyclohexapeptide dianthin G†
Abstract
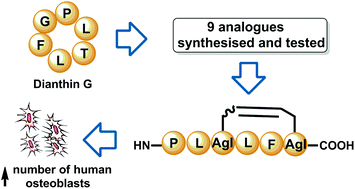
The cyclohexapeptide natural product dianthin G promotes osteoblast (bone-forming cell) proliferation in vitro at nanomolar concentrations, and is therefore considered a promising candidate for the treatment of osteoporosis. An Nα-methyl amide bond scan of dianthin G was performed to probe the effect of modifying amide bonds on osteoblast proliferation. In addition, to provide greater structural diversity, a series of dicarba dianthin G analogues was synthesised using ring closing metathesis. Dianthin G and one novel dicarba analogue increased the number of human osteoblasts and importantly they did not increase osteoclast (bone-resorbing cell) differentiation in bone marrow cells.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins
 Please wait while we load your content...
Please wait while we load your content...