Unexpected formation of π-expanded isoquinoline from anthracene possessing four electron-donating groups via the Duff reaction†
Abstract
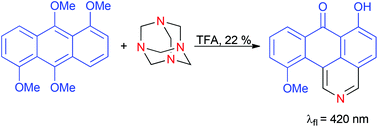
New synthetic methods leading towards π-expanded heterocycles are sought after mainly due to their promising opto-electronic properties. Subjecting 1,5,9,10-tetramethoxyanthracene to the modern Duff reaction conditions led to the formation of a compound possessing the 2-azabenzoanthrone (dibenzo[de,h]isoquinolin-7-on) skeleton instead of the expected dialdehyde. This non-typical course of reaction can be rationalized by the double electrophilic aromatic substitution at two neighboring electron-rich positions of anthracene followed by oxidation of the resulting intermediate to form a pyridine ring. Optical studies supported by the quantum chemistry calculations indicated the lack of excited-state intramolecular proton transfer (ESIPT); for energy reasons, only one tautomeric form, with a hydrogen atom bonded to one of the two nearby oxygen atoms, was populated in the electronic ground S0 and in the excited S1 states. Nonradiative depopulation of the S1 state proceeded via internal conversion stimulated by the presence of the low frequency vibrational modes. Our serendipitous discovery represents the most complex case of rearrangement of aromatic compounds under Duff reaction conditions and could help to design analogous processes. At the same time this is the simplest method for the synthesis of derivatives of 2-azabenzoanthrone.

 Please wait while we load your content...
Please wait while we load your content...