Reactivity and selectivity of the reaction of O,O-diethyl 2,4-dinitrophenyl phosphate and thionophosphate with thiols of low molecular weight†
Abstract
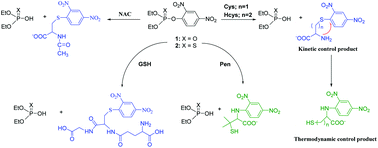
A reactivity and selectivity study of O,O-diethyl 2,4-dinitrophenyl phosphate (1) and O,O-diethyl 2,4-dinitrophenyl thionophosphate (2) with a series of thiols of low molecular weight: N-acetyl cysteine (NAC), L-cysteine (Cys), homocysteine (Hcys), glutathione (GSH), and D-penicillamine (Pen) was conducted. Results show that (i) these nucleophiles only attack at the aromatic moiety of both triester derivatives, (ii) a kinetic control product by sulfhydryl attack of thiols was observed in the reactions of both triesters with Cys and Hcys, followed by an intramolecular amine attack leading to a thermodynamic control product. The kinetic study leads to the proposal of Meisenheimer complex formation and then proton transfer to the reaction media as the mechanism of these reactions.

 Please wait while we load your content...
Please wait while we load your content...