Synthesis and biological evaluation of acylated oligorhamnoside derivatives structurally related to mezzettiaside-6 with cytotoxic activity†
Abstract
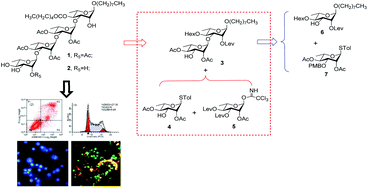
Two partially acylated oligorhamnoside derivatives 1 and 2 structurally related to the natural product mezzettiaside-6 were synthesized via a ‘2 + 1 + 1’ convergent strategy. The bioassay results showed that the introduction of the acetyl groups to the 2-position of the terminal L-rhamnose was helpful to improve in vitro cytotoxicity. Compound 1 showed both extensive in vitro cytotoxicity in tumor cell lines and potential antimultidrug resistance capability. Preliminary mechanistic studies demonstrated that compound 1 could inhibit cell growth by inducing apoptosis, arresting cell cycle progression at the S phase in K562 cells.

 Please wait while we load your content...
Please wait while we load your content...