Lighting up sugars: fluorescent BODIPY–gluco-furanose and –septanose conjugates linked by direct B–O–C bonds†
Abstract
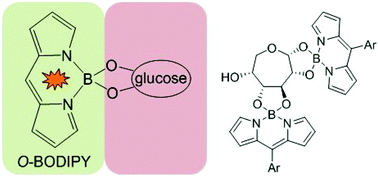
We report the first O-BODIPY–glucose conjugates, in which the sugar is directly attached to the BODIPY boron through covalent B–O–C bonds. The reaction of Cl-BODIPY with glucose in acetonitrile produced the 1 : 1 α-glucofuranose BODIPY (1), 1 : 2 α-glucofuranose BODIPY (2) and 1 : 2 α-glucoseptanose BODIPY (3) esters. Compound 3 is a rare instance of the unnatural septanose form of glucose, and the first example of a septanose borate.


 Please wait while we load your content...
Please wait while we load your content...