C2-Alkenylation of N-heteroaromatic compounds via Brønsted acid catalysis†
Abstract
Substituted heteroaromatic compounds, especially those based on pyridine, hold a privileged position within drug discovery and medicinal chemistry. However, functionalisation of the C2 position of 6-membered heteroarenes is challenging because of (a) the difficulties of installing a halogen at this site and (b) the instability of C2 heteroaryl-metal reagents. Here we show that C2-alkenylated heteroaromatics can be accessed by simple Brønsted acid catalysed union of diverse heteroarene N-oxides with alkenes. The approach is notable because (a) it is operationally simple, (b) the Brønsted acid catalyst is cheap, non-toxic and sustainable, (c) the N-oxide activator disappears during the reaction, and (d) water is the sole stoichiometric byproduct of the process. The new protocol offers orthogonal functional group tolerance to metal-catalysed methods and can be integrated easily into synthetic sequences to provide polyfunctionalised targets. In broader terms, this study demonstrates how classical organic reactivity can still be used to provide solutions to contemporary synthetic challenges that might otherwise be approached using transition metal catalysis.
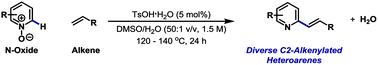
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...