2′-O-Methyl- and 2′-O-propargyl-5-methylisocytidine: synthesis, properties and impact on the isoCd–dG and the isoCd–isoGd base pairing in nucleic acids with parallel and antiparallel strand orientation†
Abstract
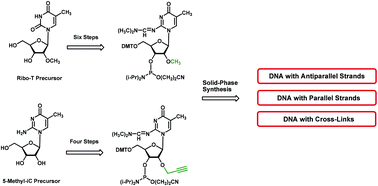
Oligonucleotides containing 2′-O-methylated 5-methylisocytidine (3) and 2′-O-propargyl-5-methylisocytidine (4) as well as the non-functionalized 5-methyl-2′-deoxyisocytidine (1b) were synthesized. MALDI-TOF mass spectra of oligonucleotides containing 1b are susceptible to a stepwise depyrimidination. In contrast, oligonucleotides incorporating 2′-O-alkylated nucleosides 3 and 4 are stable. This is supported by acid catalyzed hydrolysis experiments performed on nucleosides in solution. 2′-O-Alkylated nucleoside 3 was synthesized from 2′-O-5-dimethyluridine via tosylation, anhydro nucleoside formation and ring opening. The corresponding 4 was obtained by direct regioselective alkylation of 5-methylisocytidine (1d) with propargyl bromide under phase-transfer conditions. Both compounds were converted to phosphoramidites and employed in solid-phase oligonucleotide synthesis. Hybridization experiments resulted in duplexes with antiparallel or parallel chains. In parallel duplexes, methylation or propargylation of the 2′-hydroxyl group of isocytidine leads to destabilization while in antiparallel DNA this effect is less pronounced. 2′-O-Propargylated 4 was used to cross-link nucleosides and oligonucleotides to homodimers by a stepwise click ligation with a bifunctional azide.
 Please wait while we load your content...
Please wait while we load your content...