Improved δ-valerolactam templates for the assembly of Aβ-miniamyloids by boronic ester formation†
Abstract
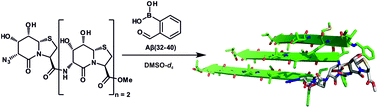
The 6,7,8,8a-cis (all-cis) substituted δ-valerolactams of type 10, 11 and 12 are high-affinity diols for boronic ester formation, superior to the corresponding 6,7-trans analogues 1, 3 and 4. X-ray and NMR structure analysis have identified the differences of the six-membered ring conformations which cause the improved esterification properties of the all-cis stereoisomers. The homooligomeric all-cis δ-valerolactams 46–48 are used as polyol templates for the self-assembly of peptidic oligomers 49–52 by dynamic covalent chemistry. The templates have a diol spacing of approximately 5 Å, suitable for the assembly of branched peptides from the quantitative reaction between the peptide of interest, 2-formylphenylboronic acid and the respective template. According to this strategy, the tetrameric Aβ-miniamyloid 52 formed spontaneously from nine individual molecules in a three-component system. A detailed NMR analysis based on the complete sequential assignment of the trimeric Aβ(32–40)-miniamyloid 51 identified its three-dimensional structure in solution.

 Please wait while we load your content...
Please wait while we load your content...