Selective inhibition of p97 by chlorinated analogues of dehydrocurvularin†
Abstract
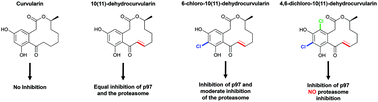
The ATPase p97 is a ubiquitin targeted segregase that uses the energy of ATP binding and hydrolysis to extract ubiquitylated substrates from biological membranes, from other proteins, or from protein complexes to carry out myriad tasks in eukaryotes. Increased p97 activity has been linked to a poor prognosis in cancer patients, making p97 an anti-neoplastic target. In the present study, we show that dehydrocurvularin (DHC) and its chlorinated variants are covalent inhibitors of p97, interfering with its ATPase activity. Interestingly, cellular studies revealed both DHC and its monochloro analogue interfere with both the proteasome and p97, whereas its dichloro analogue showed p97 specificity.

 Please wait while we load your content...
Please wait while we load your content...