Pd(0)-catalyzed cross-coupling of allyl halides with α-diazocarbonyl compounds or N-mesylhydrazones: synthesis of 1,3-diene compounds†
Abstract
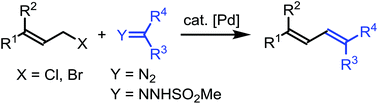
With palladium catalysis, allyl bromides or chlorides react with α-diazocarbonyl compounds or N-mesylhydrazones to afford 1,3-diene derivatives. The reaction represents a novel and efficient method for the synthesis of 1,3-butadiene derivatives. Mechanistically, the reaction is proposed to follow a pathway involving the formation of a π-allylic palladium carbene complex and subsequent migratory insertion.

 Please wait while we load your content...
Please wait while we load your content...