Efficient one-pot synthesis of amino-benzotriazolodiazocinone scaffolds via catalyst-free tandem Ugi–Huisgen reactions†
Abstract
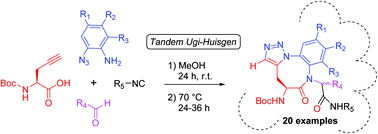
Herein we describe a catalyst-free, one-pot procedure employing an Ugi-4CR between propargyl glycine, functionalised 2-azidoanilines, different isocyanides and aldehydes, followed by a thermal azide–alkyne Huisgen cycloaddition to generate a 14-member set of amino-benzotriazolodiazocine-bearing dipeptides with multiple points of diversification and high atom economy. These structures were derivatized by means of Suzuki–Miyaura cross-coupling reactions at two positions with good to excellent yields, leading to conformationally constrained tricyclic structures. In silico and NMR conformational analysis studies demonstrated that turn conformations are adopted by these structures.

 Please wait while we load your content...
Please wait while we load your content...