5-Hydroxy-7-azaindolin-2-one, a novel hybrid of pyridinol and sunitinib: design, synthesis and cytotoxicity against cancer cells†
Abstract
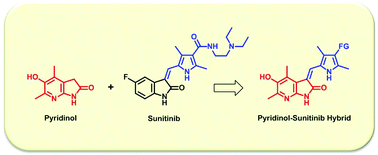
Angiogenesis plays important roles in tumor growth and metastasis. Sunitinib (Sutent®) is an antitumor agent targeting receptor tyrosine kinases which are involved in angiogenesis as well as cancer cell growth and survival. Using the pyridin-3-ol scaffold, which was previously reported as an excellent antioxidant and antiangiogenic platform, we have synthesized sunitinib mimics 6 by hybridizing bicyclic pyridinol 4 as a key scaffold and pyrrole-2-carbaldehydes 7 as side chains. Cytotoxicity assays showed that compounds 6 have comparable to better anticancer activity than sunitinib against five different cancer cell lines. In addition, compounds 6 showed even lower levels of cytotoxicity against normal cells, resulting in up to 26-fold better safety windows, than sunitinib. Signaling pathway-associated transcription factor reporter assay and western blot analyses revealed that apoptosis induction in MDA-MB-231 human breast cancer cells by 6F is mainly mediated through the p53 increase and down-regulation of phospho-signal transducer and activator of transcription 3 (STAT3) and its target gene products, cyclin D, Bcl-2, and survivin. The data strongly suggest that our hybrid compounds can provide a novel anticancer scaffold with improved and safer cytotoxicity profiles than sunitinib.
 Please wait while we load your content...
Please wait while we load your content...