On-resin Diels–Alder reaction with inverse electron demand: an efficient ligation method for complex peptides with a varying spacer to optimize cell adhesion†
Abstract
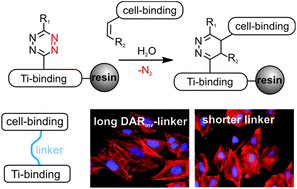
Solid phase peptide synthesis (SPPS) is the method of choice to produce peptides. Several protecting groups enable specific modifications. However, complex peptide conjugates usually require a rather demanding conjugation strategy, which is mostly performed in solution. Herein, an efficient strategy is described using an on-resin Diels–Alder reaction with inverse electron demand (DARinv). This method is compatible with the standard Fmoc/tBu strategy and is easy to monitor. As a proof of concept a titanium binding peptide was modified with a cyclic cell binding peptide (RGD) by DARinv on a solid support applying different tetrazines and alkenes. The generated bulky DARinv linkers were employed to act as the required spacer for RGD mediated cell adhesion on titanium. In vitro studies demonstrated improved cell spreading on DARinv-conjugated peptides and revealed, in combination with molecular dynamics-simulation, new insights into the design of spacers between the RGD peptide and the surface. Performing the DARinv on resin expands the toolbox of SPPS to produce complex peptide conjugates under mild, catalyst free conditions with reduced purification steps. The resulting conjugate can be effectively exploited to promote cell adhesion on biomaterials.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins
 Please wait while we load your content...
Please wait while we load your content...