A convenient method for the synthesis of α-carboxylate ester bromolactones via bromolactonization of alkenoic diesters†
Abstract
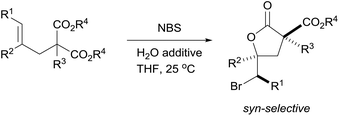
We developed a simple and efficient method to construct a variety of α-ester bromolactones in good to excellent yields with high diastereoselectivies. The protocol uses readily prepared malonate ester derivatives as substrates, water as an additive and inexpensive N-bromosuccinimide as the halogen source; no catalyst or toxic additive is required. The resulting substituted bromolactones are the fundamental units of many useful molecules.

 Please wait while we load your content...
Please wait while we load your content...