A DFT study on PBu3-catalyzed intramolecular cyclizations of N-allylic substituted α-amino nitriles for the formation of functionalized pyrrolidines: mechanisms, selectivities, and the role of catalysts†
Abstract
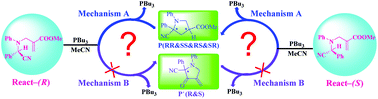
The mechanisms and chemo- and stereo-selectivities of PBu3-catalyzed intramolecular cyclizations of N-allylic substituted α-amino nitriles leading to functionalized pyrrolidines (5-endo-trig cyclization, Mechanism A) and their competing reaction leading to another kind of pyrrolidine (5-exo-trig cyclization, Mechanism B) have been investigated using density functional theory (DFT). Multiple possible reaction pathways associated with four different isomers (RR, SR, RS, and SS) for Mechanism A, and two isomers (R and S) for Mechanism B have been studied. The calculated results indicate that the Gibbs free energy barriers of Mechanism A are remarkably lower than those of Mechanism B, and the reaction pathway leading to the RS-configured product has the lowest Gibbs free energy barrier, which is in agreement with the experiments. A C–H⋯π interaction has been identified to be responsible for the favorability of RS isomers by non-covalent interaction (NCI) analysis. Moreover, global reaction indexes (GRIs) and NBO analyses confirm that PBu3 acts as a Lewis base to strengthen the nucleophilicity of the reaction active site. The mechanistic insights gained in the present study should be valuable for the rational design of effective organocatalysts for this kind of reaction with high chemo- and stereo-selectivities.

 Please wait while we load your content...
Please wait while we load your content...