Synthesis and biological evaluation of peptide-conjugated phthalocyanine photosensitizers with highly hydrophilic modifications†
Abstract
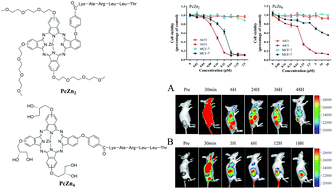
The selectivity of targeted photosensitizer delivery is a constant challenge for photodynamic therapy (PDT). Herein, with the aim of optimizing the affinity, selectivity and activity of peptide-conjugated photosensitizers for PDT therapy and fluorescence imaging, we designed a solid-phase strategy for the efficient synthesis of peptide–phthalocyanine (Pc) conjugates with two types of highly hydrophilic modifications to the Pc rings. The peptide conjugation clearly increased the photodynamic efficacy and selectivity of Pcs against cancer cells with different receptor expression levels. A highly hydrophilic modification to the Pc rings can block non-specific interactions between the Pcs and biological molecules while minimally influencing peptide ligand affinity with target receptors. Compared with the triethyleneglycol monomethyl ether group modification, the glycerol group modification exhibited a stronger capability to decrease the Pc aggregation tendency in aqueous medium, reduce non-specific binding and non-specific, light-induced cytotoxicity towards cells with low receptor expression in vitro; increased targeting selectivity was observed in the in vivo distribution experiments via the obvious reduction of background signals. Highly hydrophilic modifications to the Pc rings may be very useful for improving targeting selectivity for PDT with the construction of peptide-conjugated photosensitizers.

 Please wait while we load your content...
Please wait while we load your content...