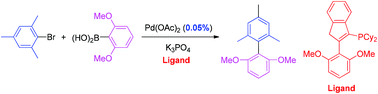
An efficient indenyl-derived phosphine ligand for the Suzuki–Miyaura coupling of sterically hindered aryl halides†
Abstract
An air-stable aryl substituted indenyl phosphine used in combination with Pd(OAc)2 provides a highly efficient catalyst for the Suzuki–Miyaura cross-coupling reaction of sterically hindered aryl halides with aryl boronic acids.

 Please wait while we load your content...
Please wait while we load your content...