Synthesis, radiolabeling with fluorine-18 and preliminary in vivo evaluation of a heparan sulphate mimetic as potent angiogenesis and heparanase inhibitor for cancer applications†
Abstract
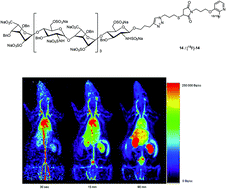
Heparan Sulfate (HS) mimetics are able to block crucial interactions of the components of the extracellular matrix in angiogenic processes and as such, represent a valuable class of original candidates for cancer therapy. Here we first report the synthesis and in vitro angiogenic inhibition properties of a conjugated, novel and rationally-designed octasaccharide-based HS mimetic. We also herein report its labeling with fluorine-18 and present the preliminary in vivo Positron Emission Tomography imaging data in rats. This constitutes one of the rare examples of labeling and in vivo evaluation of a synthetic, polysaccharide-based, macromolecule.

 Please wait while we load your content...
Please wait while we load your content...