Stereocontrolled synthesis of rosuvastatin calcium via iodine chloride-induced intramolecular cyclization†
Abstract
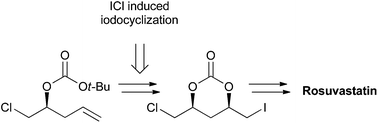
A novel, stereoselective approach towards rosuvastatin calcium from the known (S)-homoallylic alcohol has been developed. The synthesis is highlighted by a regio- and stereocontrolled ICl-induced intramolecular cyclization of chiral homoallylic carbonate to deliver the C6-formyl statin side chain with a syn-1,3-diol moiety. An improved synthesis of the rosuvastatin pyrimidine core moiety is also included. Moreover, this methodology is useful in the asymmetric synthesis of structural variants of statins such as pitavastatin calcium and atorvastatin calcium and their related analogs.

 Please wait while we load your content...
Please wait while we load your content...