NIR bacteriochlorin chromophores accessed by Heck and Sonogashira cross-coupling reactions on a tetrabromobacteriochlorin derivative†
Abstract
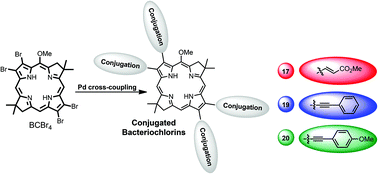
The synthesis of a new tetrabromobacteriochlorin BCBr4 is reported having the 3,4-dibromo-1H-pyrrole-2-carbaldehyde (10) as the major precursor. The BCBr4 was successfully employed in Pd cross-coupling reactions with methyl acrylate, phenyl acetylene and 4-ethynylanisole. In all three cases, the desired tetra-coupled products were obtained in good to excellent yields, and present a significant red shift in the UV-Vis bands above 800 nm. DFT and TD-DFT theoretical analyses of the NIR bacteriochlorin chromophores were performed in order to evaluate the effect of β substitution on their electronic structures.

 Please wait while we load your content...
Please wait while we load your content...