The chemical synthesis of aryltetralin glycosides
Abstract
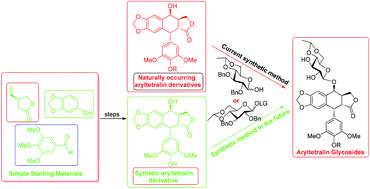
Led by etoposide and teniposide, the synthesis of aryltetralin glycosides has been experiencing flourishing development in the past five decades. Herein, a review focusing on the total synthesis of aryltetralin glycosides is provided. The main body of this review is composed of two parts, one is the enantioselective synthesis of aryltetralin derivatives and the other one is the construction of key glycosidic linkages. In each part the contents are organised based on the different strategies or protocols applied in the original documents. The total synthesis of aryltetralin glycosides represents the developing direction of this field, and sooner or later will replace the currently applied semi-total synthesis method, using the aglycon residue acquired directly from natural sources. This account provides a comprehensive and deep insight into the field of aryltetralin glycoside synthesis for chemists who have the intention of committing themselves to the development of aryltetralin glycoside medicine.

 Please wait while we load your content...
Please wait while we load your content...