Cyclopent-2-enylaluminium as allylzinc precursor for the diastereoselective allylmetallation of non-racemic imines: applications to the synthesis of enantiomerically enriched heterocycles†
Abstract
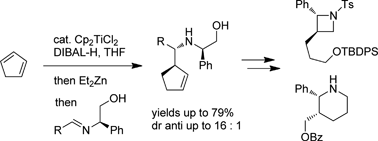
The generation of cyclopent-2-enylzinc from cyclopentadiene based on a titanium-catalyzed hydroalumination/transmetallation sequence is described. Applied to the allylmetallation of phenylglycinol-derived imines, this sequence leads to homoallylic amines with moderate to good stereoselectivities. The synthesis of disubstituted azetidines and piperidines illustrates the potential of the method.

 Please wait while we load your content...
Please wait while we load your content...